For the preparation of cell culture media there is a virtually endless choice in recipes with different bulk densities, particle sizes or flow properties. Once the right mixture is found, various parameters become important, as Hermann Schmidt, Operations Director Pharma & Lab Division at Hosokawa Alpine, explains. “On the one hand, the mixing ratio must be replicable to guarantee exact results. In addition, there must be no contamination in the mixing process itself - unwanted contact with oxygen, moisture or germs in the environment must be avoided.”
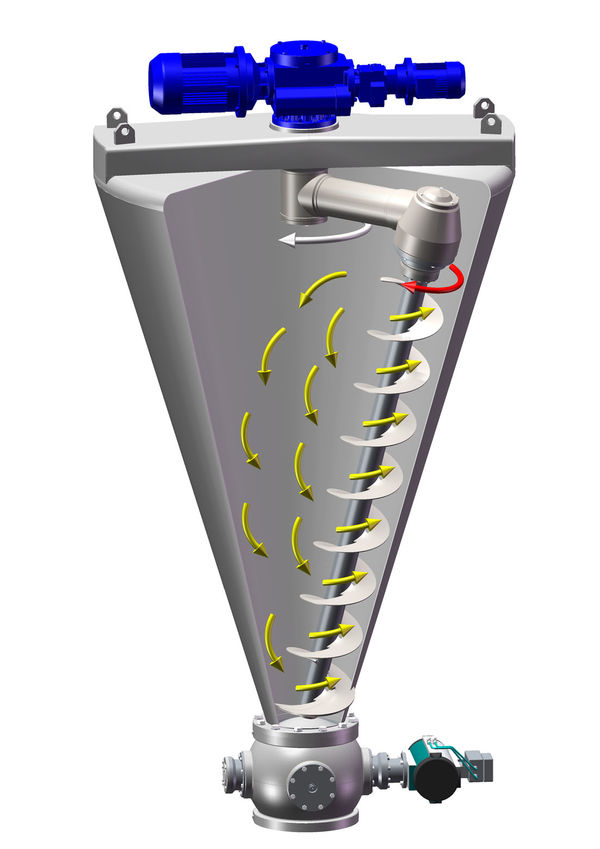
Throughout the manufacturing process, a high degree of automation not only ensures safety and reliability, but also makes it possible to produce a wide variety of media and batch sizes on one system. This flexibility must be guaranteed because new batches with different formulations are produced daily for the preparation of cell cultures. The micronisation takes place in Hosokawa Alpine’s UPZ fine impact mill. With its variety of grinding inserts, this mill type can create product finenesses from 90 % < 150 µm up to < 20 µm.
“In the production of cell culture media, the pin discs are ideal for finely micronised and homogeneous products. Due to the combination of the fitting process parameters, the fine product features a steep particle size distribution and good mixing properties”, as Hermann Schmidt explains. Another suitable solution for the formulation of special cell culture media is the cryogenic processing by Hosokawa Alpine Contraplex pin mill, which enables gentle micronisation of sensitive raw materials.


Recommend article